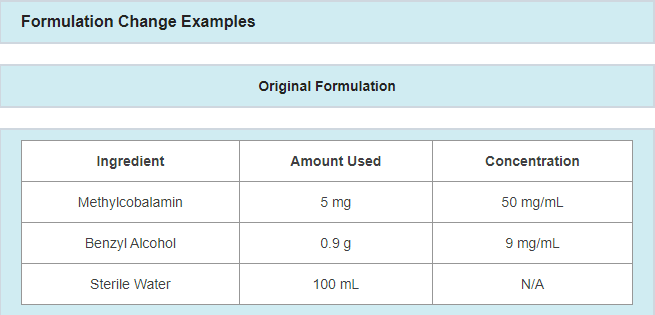
A Master Formulation Record (MFR) is a detailed record of procedures that describes how the drug product is to be prepared. This documentation is an important component of regulatory compliance and effective process control.
According to USP <795> and <797>, a MFR must be created for:
- Each unique formulation of a compounded nonsterile preparation (CSNP)
- Compounded sterile preparations (CSP) for more than one patient
- CSP prepared from nonsterile ingredient(s)
Any changes or alterations to the MFR must be approved and documented according to the facility’s SOPs.
Visit USP <795> and <797> for more information on a Master Formulation Record and its required contents.
Master Formulation Records and Quality Control Testing
Master Formulation Records are essential to providing testing laboratories with information on how the drug product is prepared. It gives a thorough view of your pharmacy's processes and aids ARL in providing high-quality results.
Submitting an MFR with every sample helps improve the on-time delivery of test results to your pharmacy and reduces potential out-of-specification (OOS) investigations.
ARL uses the MFR to:
- Know which diluents and methods to use to extract API for testing
- Establish method suitability to prove the test method is suitable for its intended use
- Match unique formulations to corresponding unique formulation identification numbers
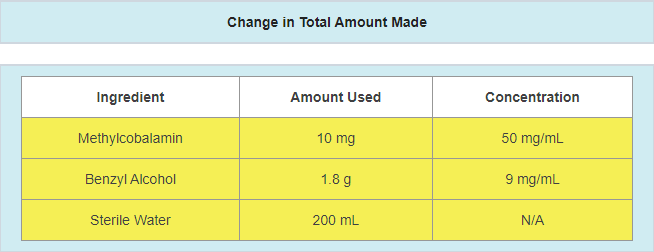
Pharmacists must update MFRs with any changes or alterations to the compounded preparation.
Changes or alterations include, but are not limited to:
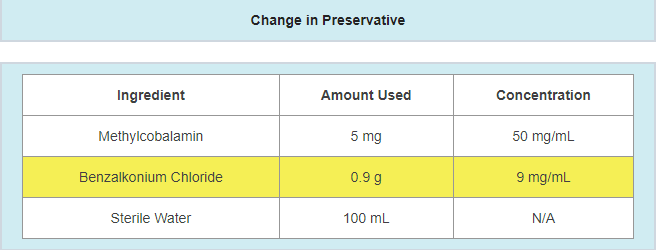
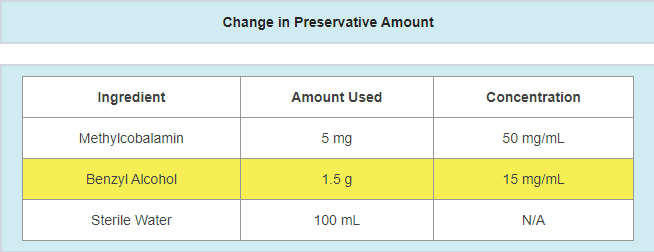
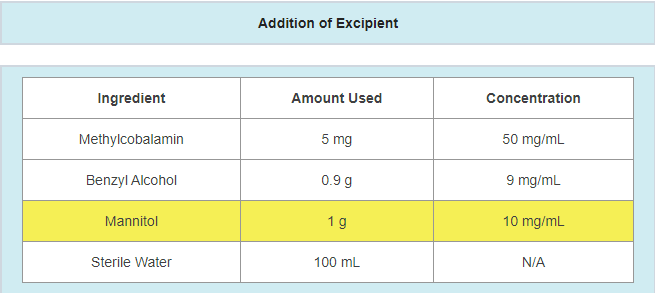
- A change in preservative or preservative amount
- Example: updating preservative and/or preservative amount in response to a failing antimicrobial effectiveness test
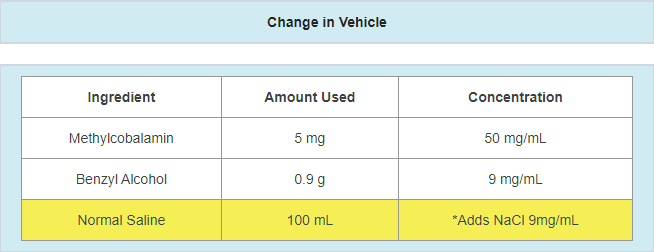
- A change in the vehicle
- Example: vehicle supplier changes due to availability
- A change in suppliers where the new supplier’s formulation does not match the old supplier
- Example: 0.9% normal saline vs. 0.45% normal saline
- Example: salt form vs. non-salt form
- Example: preservative-free vs. preserved vehicle
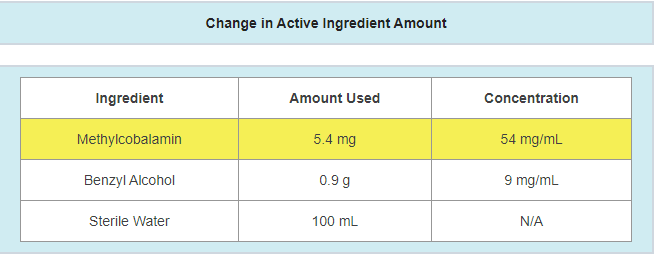
- A change in the amount of active ingredients used
- Example: active ingredient updated due to a potency failure
- A change in notes
- A change in active ingredient starting material – raw material vs. triturate vs. commercial product
- A change in a lot of active ingredients received from a supplier
- Example: purity factor or water content
It is crucial to document the compounding process of each drug product by creating a Compounding Record (CR) in addition to the Master Formula Record (MFR). It is also important to record the balance quantities in a CR. The MFR can be used as a reference while creating a CR. One way to do this is by incorporating spaces in the MFR to fill in the necessary information to complete the compounding record.
Visit USP <795> and <797> for more information on a compounding record and its required contents.
Submitting MFRs and CRs can help identify and resolve discrepancies during sample receiving, test analysis, and/or reporting of the results. Thus, reducing the need for additional client communication to answer questions that might arise.
For more information about submitting Master Formulation Records, contact ARL at 800-393-1595 or info@arlok.com.