What is the difference between Potency and Stability?
It’s important to understand the purpose of each test to identify the difference between potency and stability.
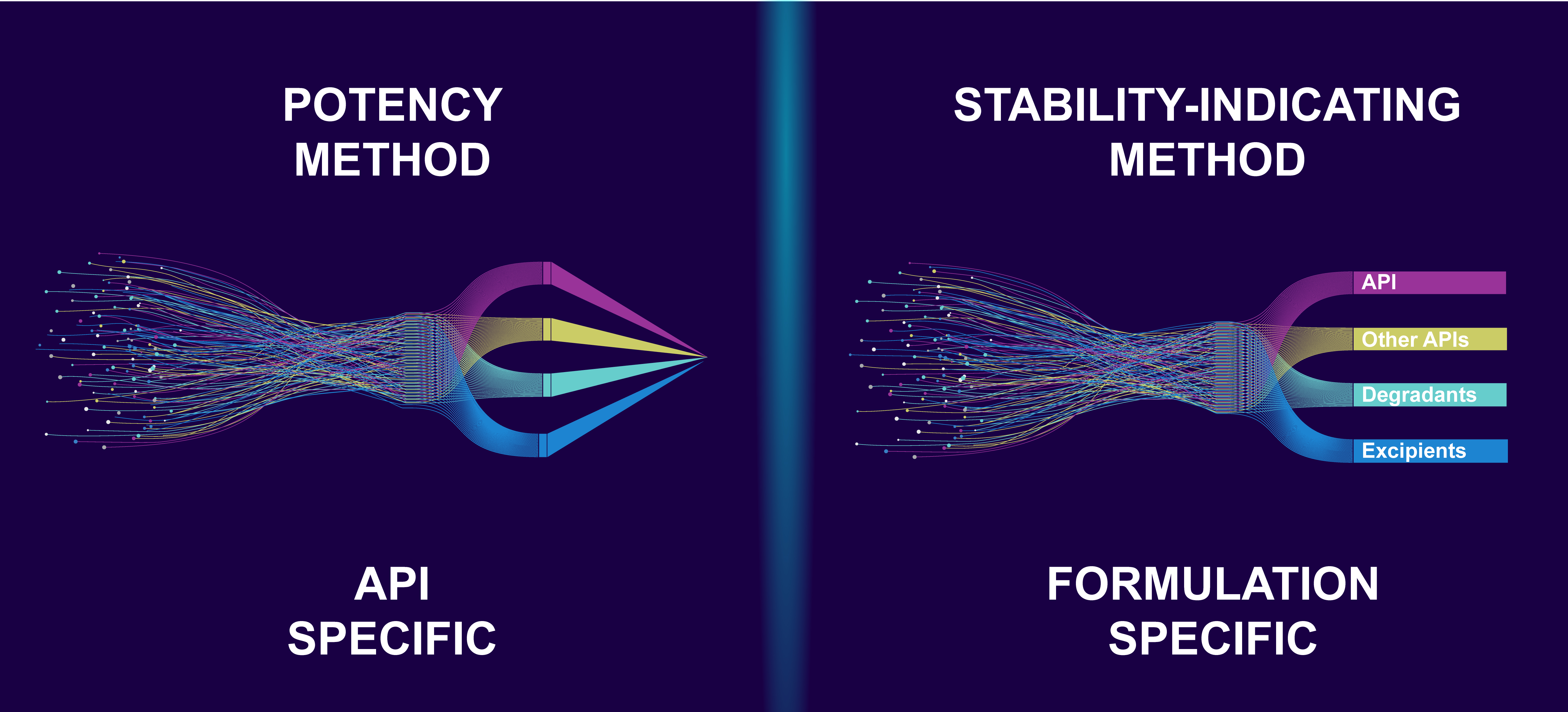
Potency vs. Stability Testing
Potency testing verifies that the active pharmaceutical ingredient matches the identity and strength stated on the label. This essential quality check is used for formulation development, process and personnel qualification, and lot release. It is typically performed within a few days after compounding and provides a real-time assessment of the drug product’s quality.
Stability testing evaluates several factors of a compounded drug product's formulation to determine a beyond-use date (BUD). It is typically performed after product development, and takes weeks to months to evaluate.
API Specific vs. Formulation Specific Methods
When ARL establishes a non-validated potency analytical method, the active pharmaceutical ingredient (API), not the specific formulation, is evaluated. The laboratory uses system suitability to verify that the instrument's resolution and repeatability are adequate for analysis to provide reliable results. The analytical method is then used on API-specific lots for release testing. If an analytical challenge is encountered during potency testing for unique formulations, ARL will optimize the method and document changes to achieve accurate results.
When ARL develops a validated stability-indicating analytical method, the specific formulation is evaluated. At the beginning of method development, stress studies are conducted using acid and base, oxidation, heat, humidity, and light to quickly create degradants of the API and excipients that may appear during stability testing. Before it can be considered fully developed, the analytical method must demonstrate its ability to effectively separate the API from degradants, impurities, and excipients. Once the method is established, it must undergo validation for accuracy, linearity, precision, range, specificity, and system suitability. This validation is crucial so that the method can be used to assess the stability of the drug product and assign appropriate BUDs. Stability-indicating analytical methods may also be used on formulation-specific lots for release testing.
ARL Certificates of Analysis and Test Methods
If a drug product is tested by a non-validated potency analytical method, ARL's certificate of analysis will have an instrument (HPLC) listed as the test method and a statement “the potency method(s) used for testing passed system suitability requirements per ARL SOP AMP-012 for non-GMP analysis. Product specific method validation is not available for the sample and specification(s) are for informational purposes only.”
If a drug product is tested by a validated stability-indicating analytical method, ARL's certificate of analysis will list a method number (e.g., AMI-1234) as the test method, indicating the product was tested using the formulation-specific method.
For more information on potency and stability, visit ARL's Education Center.
Click here to request a stability-indicating analytical method quote.
