Importance of Forced Degradation in Stability-Indicating Methods
Pharmacists often want to assign a beyond-use date (BUD) on a drug product longer than USP guidelines to increase compounding efficiencies and reduce drug waste. For accurate BUD assignment, Stability-indicating methods are recommended as opposed to Potency methods. Stability-indicating methods are specifically designed to separate drug degradants from non-degraded drug (for more information about the difference between the two methods, see Stability vs. Potency testing article).
Forced degradation uses stress conditions to create degradants in a drug product. This is required to demonstrate specificity in a stability indicating method and ensure an active ingredient’s chemical stability over time.
Quantification of analytes
Potency and stability are generally measured by a (U)HPLC coupled with a detector (overview of HPLC technology). These methods work in two steps. First, the method must separate the ingredients in the drug product, and then measure a signal for detected compounds. The result is translated into a chromatogram (Figure 1), a plot that gives the signal measured over time. If the method is well developed, each peak on the chromatogram corresponds to one compound. In this figure the test method is stability indicating because it separates Edetate Disodium from its degradant.
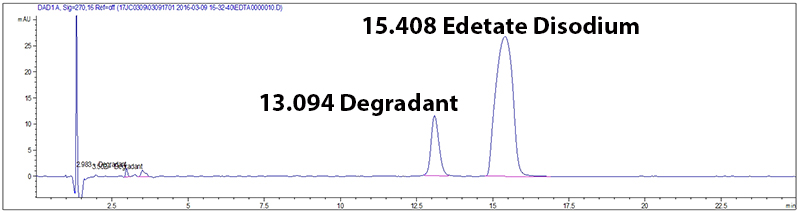
Figure 1: Example of a chromatogram
If the method is poorly designed, step one does not occur and the chromatogram displays only one peak. When the two different compounds are combined into one peak, the test provides an incorrect potency value. An incorrect potency result would lead to an inappropriate BUD assignment. To avoid this, all ingredients in the compounded preparation and drug degradants must be separated from the drug. Potency methods are designed to separate other ingredients in a drug product, but only stability indicating methods are designed to separate degradants.
Generation of degradants during method development
To develop a Stability-indicating method, ARL’s chemists create degradants that are likely to be formed as the compounded preparation ages. This is done through forced degradation studies. These studies involve subjecting the drug product and placebo to multiple stress conditions as recommended by these institutions:
- USP in USP<1225>: Validation of Compendial Procedures
SPECIFICITY
“If impurity or degradation product standards are unavailable, specificity may be demonstrated by comparing the test products to a second well –characterized procedure (e.g., a Pharmacopeial or other validated procedure). These comparisons should include samples stored under relevant stress conditions (e.g., light, heat, humidity, acid/base hydrolysis, and oxidation). - The FDA in FDA Guidance for Industry Analytical Procedures and Methods Validation
Stress Studies: “Degradation information obtained from stress studies (e.g., products of acid and base hydrolysis, thermal degradation, photolysis, oxidation) for the drug substance and for the active ingredient in the drug product should be provided to demonstrate the specificity of the assay and analytical procedures for impurities […] do not interfere with the quantitation of the active ingredient.” - ICH in ICH Validation of Analytical Procedures Text and Methodology Q2(R1)
1.2.2 “Specificity may be demonstrated […]. This should include samples stored under relevant stress conditions: light, heat, humidity, acid/base hydrolysis and oxidation.”
Per these organizations, ARL performs six forced degradation studies when developing stability indicating methods. Those are:
- Acid hydrolysis
- Base hydrolysis
- Thermal degradation
- Humidity
- Photolysis
- Oxidation
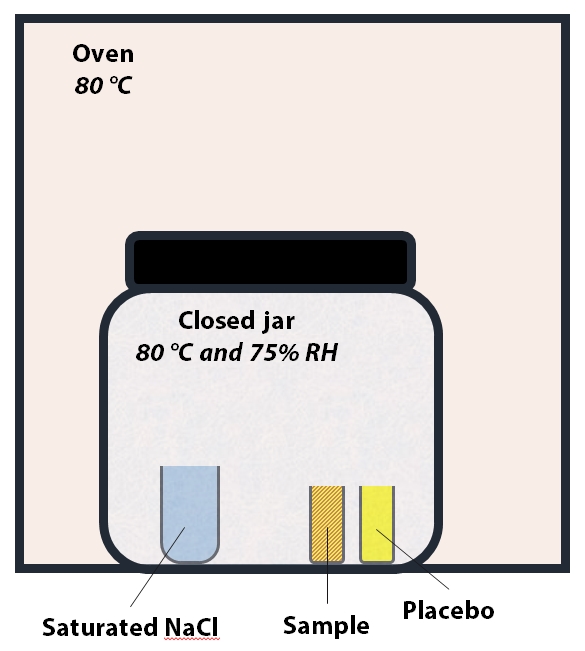
Figure 2: Set up for Heat and Humidity stress. The temperature inside the jar is 80 °C and relative humidity is ~75%
Once all stress conditions are performed, each stressed sample and controls are tested on the HPLC to see what degradation peaks are created. It is critical to examine each stress condition because different stress conditions can create different degradants. Notice in Figure 3, UV light stress generated different degradants than heat and humidity stress. The different degradants are circled in red.
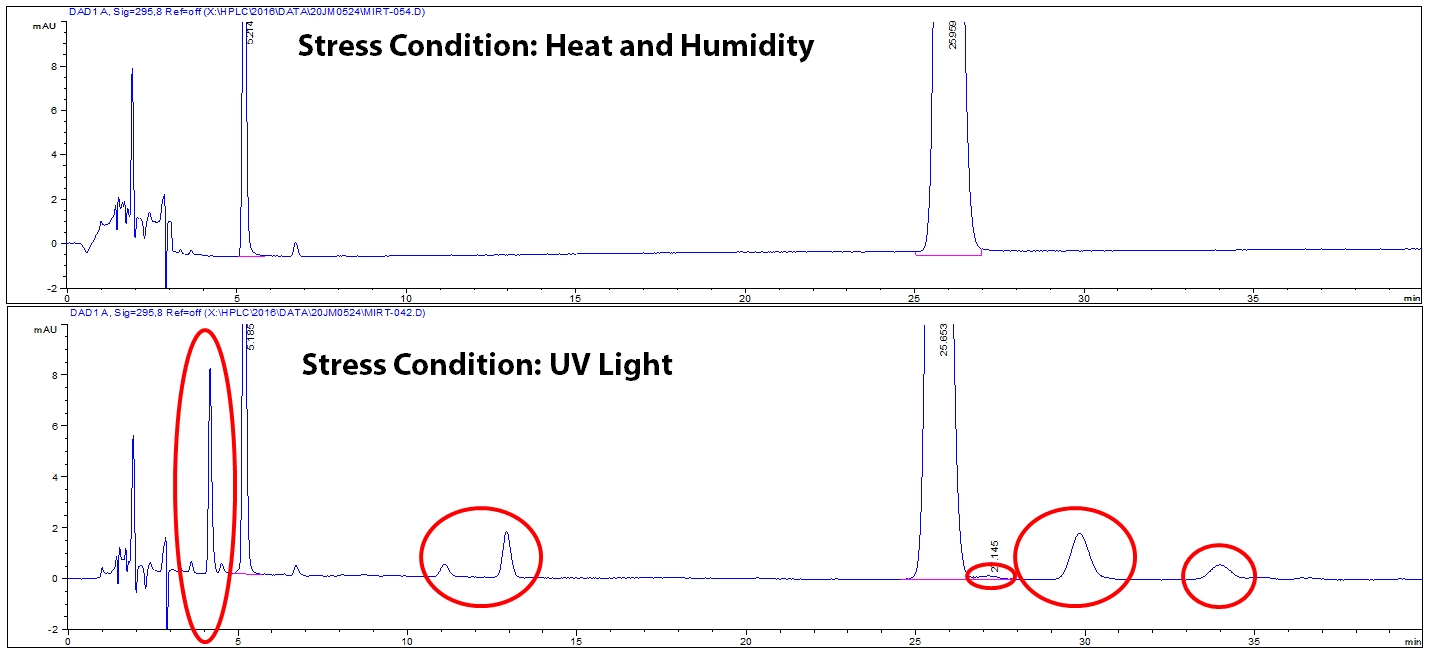
Figure 3: Example of degradation chromatograms for two stress conditions.
ARL chemists develop a test method that separates all degradants created by the stress studies from the drug. Once this separation is achieved, the method is considered stability indicating and can now be validated. Method validation ensures the test method is accurate, precise, has a linear response and is robust to small changes. After the stability indicating test method is validated, the test method is considered reliable, meaningful, and specific and the stability study can be conducted to determine the stability and beyond-use date of a product.
