According to USP <71> Sterility Tests, membrane filtration is the sterility test method of choice for filterable pharmaceutical products. Membrane filtration refers to either an open membrane filtration (OMF) or closed membrane filtration system. Not all sterility test systems are the same. It is important pharmacists know the differences between the two systems to reduce the risk of false positive results, costly investigations, and batch loss.
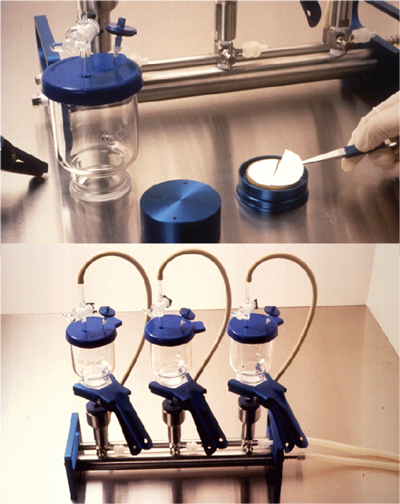
Open Membrane Filtration System
Open Membrane Filtration (OMF)
The OMF testing process passes the drug product through an open funnel containing a filter. Once appropriate filter rinsing is complete, the filter is removed, cut in half, and placed individually into each media type used for sterility testing; tryptic soy broth (TSB) and fluid thioglycollate medium (FTG or FTM). Incubation occurs at the appropriate temperatures and duration. The required handling of the filters greatly increases the risk of contaminating the sterility test sample during the sterility test procedure.
Risks of using OMF:
- Sterile drug and sterility test filter are exposed to test environment increasing false positive results
- Drug product sample may pass around the removable filter and not through it causing a false negative
- Test operator has direct contact with filter membrane and must carefully cut and manipulate filter using scissors and tweezers to avoid contamination
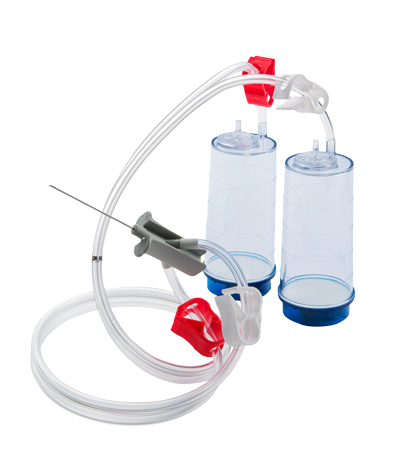
Closed Membrane Filtration
Sterility testing via closed membrane filtration is the most reliable method. It utilizes a dual canister system, where each canister contains its own 0.45 micron filter. Both canisters are attached to a single needle via sterile tubing. The test is carried out by creating a vacuum in the canisters, which allows the microbiologist to withdraw sample directly from the drug product being testing using the attached needle. The sample is then automatically split between both canisters and passes through both filters. Following the appropriate filter rinse, each media type (TSB & FTG) are individually added into the separate canisters. The tubing is sealed, and each canister is incubated at the appropriate temperatures and duration. Closed membrane filtration uses a secure system to provide high-quality sterility test results.
Benefits of using closed membrane filtration:
- The sealed drug product is never opened or pooled before filtration
- The filter itself is never opened or exposed to the test environment decreasing the risk of false positive results
- The filter is sealed in the canister reducing the risk of false negative results
- The test operator cannot touch or manipulate the filter membrane decreasing the risk of contamination
- All materials used are pre-sterilized and assembled, reducing possibility of test fault or contamination of testing materials by the laboratory
- The needle (attached to the filter canister unit) allows analysts to withdraw product for testing in the same way health professionals withdraw the product with a syringe
Sterility testing is an integral part of routine quality control and product release. It is important pharmacists use closed membrane filtration, the most reliable method, to reduce the risk of false positive results, prevent costly investigations and batch loss, and protect their patients from false negatives. ARL Bio Pharma only utilizes pre-sterilized, ready-to-use, closed membrane filtration canisters to ensure the highest quality sterility test for filterable products.
For more information about sterility testing, contact ARL at 800-393-1595 or info@arlok.com
