
A sterility test detects microbial contamination and provides data to determine whether a product is ready for release. A “Sterile” result indicates no contaminating microorganism is found in the sample. A “Not Sterile” result indicates microbial growth, and the product examined does not comply with the test for sterility, unless it is demonstrated that the test is invalid for causes unrelated to the product examined.
Sterility Test and Microbial Growth
Trained microbiologists examine the sterility test sample for microbial growth using the following conditions:
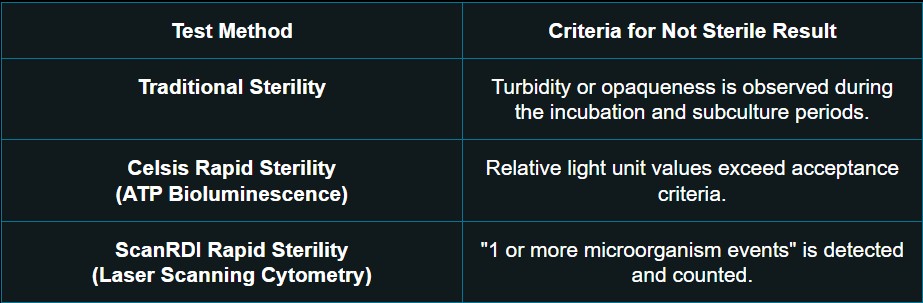
Once evidence of microbial growth is found, the test is out-of-specification (OOS), and an investigation is required to determine the cause of the test failure.
Possible Causes of Sterility Test Failure
- Compromised container integrity
- Improper aseptic processing and facilities
- Invalid sterilization processes
- Laboratory error
ARL Bio Pharma Sterility OOS Investigation Process
An OOS investigation is conducted for every sterility test failure. ARL Bio Pharma's investigation includes a detailed examination of:
- Sterility test method
- Environmental monitoring (EM) data
- Sanitization logs for all ISO environments
- DNA sequencing reports, if applicable
- Deviation reports, if applicable
- Negative control data
- Potential retest results, if the test meets invalidation criteria
Criteria for Invalidating a Sterility Test Failure
A traditional sterility or Celsis rapid sterility test may be considered invalid only if one or more of the following conditions are fulfilled:
- A species-level microbial identification match between EM isolate(s) collected during the applicable testing shift and the microorganism isolate from the sterility test.
- Evidence that the testing procedure used during the test in question reveals a fault.
A ScanRDI rapid sterility test may be considered invalid only if both of the conditions are fulfilled.
If the test is declared invalid, it is repeated with the same lot and number of samples as in the original test. If no evidence of microbial growth is found in the repeat test, the product examined complies with the sterility test. If microbial growth is found in the repeat test, the product examined does not comply with the sterility test.
Next Steps
While a species-level match meets a portion of the USP 71 invalidation requirements, a sterility failure should prompt an investigation at the facility where the product was prepared. This investigation should take place at the same time as the laboratory investigation and include a review of:
- Compounding sterility assurance practices
- Aseptic processing practices
- Equipment qualifications
- Sterilization validations
- Environmental monitoring
- Personnel training
Pharmacies and outsourcing facilities should review all the data, including ARL's findings, to determine how to proceed after a sterility test fails.
For more information, see USP chapters:
- USP 71 Sterility Tests
- USP 1207 Package Integrity Evaluation – Sterile Products
- USP 1211 Sterility Assurance
- USP 1229 Sterilization of Compendial Articles
