Personnel Qualification and Environmental Monitoring Requirements for Compounded Sterile Preparations (CSPs) for Category 3.
Category 3:
- Have additional requirements that must be met at all times
- May be assigned a BUD longer than established for Category 2 CSPs, up to 180 day
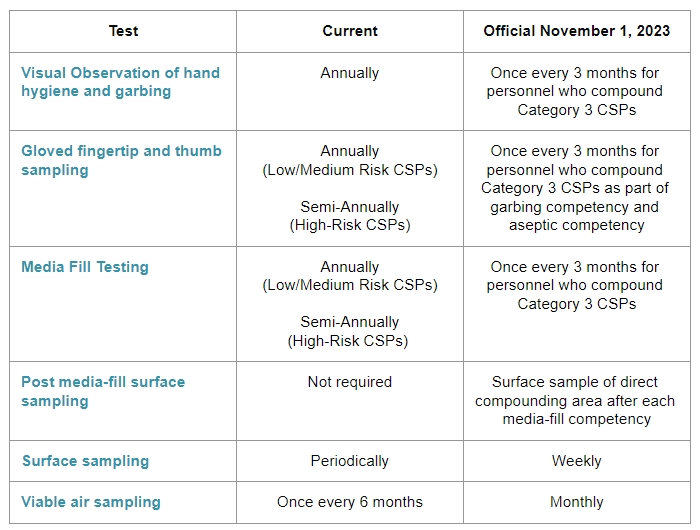
Visual Observation of hand hygiene and garbing: This is a competency evaluation of hand hygiene, garbing procedures, and gloved fingertip and thumb sampling of both hands.
Gloved fingertip and thumb sampling:
- This test provides data on any microorganisms present on the technician's gloves during compounding.
- Press gloved fingertips onto one agar plate per hand after compounding. Label plate with all relevant information including, but not limited to, staff members name, date, and time of sampling.
- Send plates to ARL for incubating, counting of colony forming units (CFUs), and reporting of a Certificate of Analysis (COA).
- Document test results.
- Determine if any additional actions are required including growth promotion of the media to prove the media was capable of detecting growth, identification of any organisms recovered, retraining of staff, etc.
- Repeat as necessary.
Media Fill Testing:
- This test assesses the technician's ability to compound aseptically.
- Personnel should perform the media fill test under the most stressful and challenging conditions at the pharmacy. A simulated compounding operation is conducted with microbial growth media in place of drug components and/or products.
- Send media fill containers to ARL for incubation and observation, and reporting of a Certificate of Analysis (COA).
- Document test results.
- Determine if any additional actions are required including growth promotion of the media to prove the media was capable of detecting growth, identification of any organisms recovered, retraining of staff, etc.
- Repeat as necessary.
Post media-fill surface sampling test:
- This test assesses the technician's ability to compound aseptically.
- At the completion of the media fill qualification procedure, the surfaces of the compounding area are sampled using agar press plates.
- Send plates to ARL for incubating, counting of colony forming units (CFUs), and reporting of a Certificate of Analysis (COA).
- Document test results.
- Determine if any additional actions are required including growth promotion of the media to prove the media was capable of detecting growth, identification of any organisms recovered, retraining of staff, etc.
- Repeat as necessary.
Surface sampling:
- This test monitors surfaces for viable particles.
- At the conclusion of a compounding session, agar press plates are used to sample the surfaces of the compounding space.
- Send plates to ARL for incubating, counting of colony forming units (CFUs), and reporting of a Certificate of Analysis (COA).
- Document test results.
- Determine if any additional actions are required including growth promotion of the media to prove the media was capable of detecting growth, identification of any organisms recovered, retraining of staff, etc.
- Repeat as necessary.
Viable air sampling:
- This test monitors air quality for viable airborne particles.
- Follow manufacturer's instructions for operation of the active air sampling device, including placement of media. Test at least 1 cubic meter or 1000 L of air from each location sampled.
- Send plates to ARL for incubating, counting of colony forming units (CFUs), and reporting of a Certificate of Analysis (COA).
- Document test results.
- Determine if any additional actions are required including growth promotion of the media to prove the media was capable of detecting growth, identification of any organisms recovered, retraining of staff, etc.
- Repeat as necessary.
