What is particulate matter and why is testing performed?
Particulate matter is unwanted solid material in solutions for injection. Particle counts that exceed USP limits in intravenous drug products could block blood flow inside the patient's body causing paralysis or death.
Particulate matter testing is a good indicator of the quality of a manufacturing process and the containers used. If the containers are not particle free on the inside, this will introduce particulates into the drug product. If the instrumentation used is not clean, unwanted solid matter could end up in a final drug product and cause patient harm.
Where does particulate matter come from?
Particulate matter comes from three sources:
- Intrinsic stems from the internal manufacturing process. This can be introduced in the drug formulation, packaging or container, and poor cleaning systems. Examples include glass from inside containers, stainless steel from container closure systems, or rubber from the stoppers.
- Extrinsic stems from the external production environment and human error. This can be introduced during improper gowning in a cleanroom and other external behavior. Examples include clothing, hair, metal, plastic, or paint that fall into sample containers.
- Inherent stems from a product’s characteristic that may contain a small level of unintended particulate matter, even after careful formulation
How is particulate matter tested?
United States Pharmacopeia has three main chapters that describe testing methods and specifications for particulate matter:
- USP <787> Subvisible Particulate Matter in Therapeutic Protein Injections
- USP <788> Particulate Matter in Injections
- USP <789> Particulate Matter in Ophthalmic Solutions
USP also has chapters that give broader information and relate to the test methodology:
- USP <771> Ophthalmic Products - Quality Tests
- USP <1788> Methods for the Determination of Subvisible Particulate Matter
- USP <1788.1> Light Obscuration Method for the Determination of Subvisible Particulate Matter
- USP <1788.2> Membrane Microscope Method for the Determination of Subvisible Particulate Matter
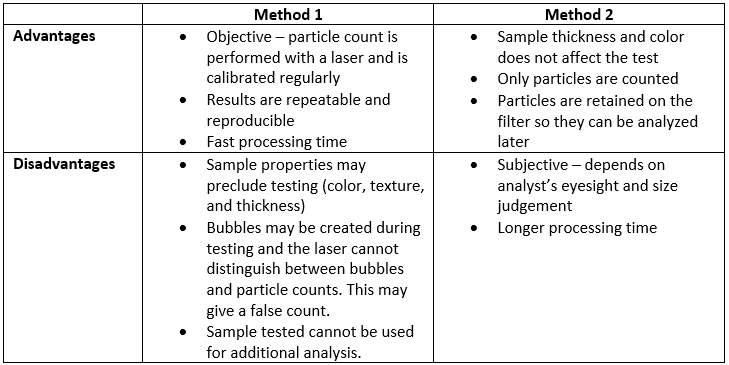
ARL Bio Pharma uses validated methodology to test particulate matter samples. Our laboratory uses two methods to test for particulate matter: Method 1 and Method 2. There are advantages and disadvantages to each method. ARL determines which method to use based on sample type.
Method 1 uses a light obscuration particle counter to test the sample. A probe goes into the sample container. The sample travels up through the laser’s path and particles are counted. When testing is complete, the sample is discarded out of the system to a waste container
Method 2 uses a microscope, and an analyst counts the particles. The particles present in a sample are collected on a membrane filter and then viewed under a microscope for counting. The analyst uses a graticule inside the microscope lens to determine the size and count of the particles based on USP criteria.
What are the USP Particle Size Limits for Light Obscuration and Microscopy?
USP <787> Subvisible Particulate Matter in Therapeutic Protein Injections

USP <788> Particulate Matter in Injections
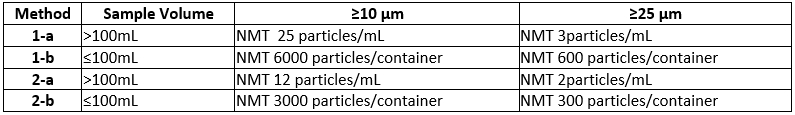
USP <789> Particulate Matter in Ophthalmic Solutions

For more information on particulate matter testing, contact (800) 393-1595 or info@arlok.com
