Antimicrobial preservatives are excipients added to aqueous pharmaceutical products.
Nonsterile dosage forms may have preservatives added to protect them from the growth of microorganisms inadvertently introduced during or after the compounding process. USP <795> Pharmaceutical Compounding—Nonsterile Preparations requires passing USP <51> Antimicrobial Effectiveness Testing data on aqueous, compounded non-sterile preparations if extending the beyond use date past the limits in USP <795> Table 4. USP defines an aqueous preparation as one with a water activity greater than 0.6.
Sterile dosage forms packaged in multiple-dose containers have preservatives added to inhibit the growth of microorganisms that may be introduced from repeatedly withdrawing individual doses. USP <797> Pharmaceutical Compounding—Sterile Preparations requires passing antimicrobial effectiveness testing data on aqueous, multiple-dose compounded sterile products.
Both <795> and <797> state antimicrobial effectiveness testing must be conducted and passed once for each formulation in accordance with USP <51> for the particular container closure system in which it will be packaged. For a product to pass antimicrobial effectiveness testing, formulations containing preservatives are expected to meet minimal efficacy standards, whether packaged as multidose or unit doses. The compounder may rely on results from an FDA-registered facility or published in peer-reviewed literature sources if the formulation (including any preservative) and container closure materials of composition are the same as those tested (unless a bracketing study is performed). When a bracketing study is performed, antimicrobial effectiveness testing may be performed on a low concentration and on a high concentration of the active ingredient in the formulation to establish preservative effectiveness across various strengths of the same formulation (e.g., bracketing). The concentration of all other ingredients (including preservatives) must fall within the bracketed range.
FDA requires 503B Outsourcing Facilities to have passing antimicrobial effectiveness testing data on sterile drug products labeled as multiple-dose and for aqueous non-sterile products labeled as multiple-dose. If antimicrobial effectiveness testing was previously performed using the subject formulation and container-closure system, preservative content testing may be used in lieu of a full antimicrobial effectiveness study. Appropriate specifications for aqueous drug products labeled as multiple-dose include assurances that the product is adequately self-preserving or contains appropriate preservative content to limit microbial proliferation of microorganisms and assures the product maintains its quality and purity for each dose. Antimicrobial effectiveness studies only need to be conducted once for each formulation and container-closure system, and a bracketing or matrixing approach can be considered to minimize the amount of testing.
Testing of Products
USP <51> is designed to challenge the antimicrobial properties of the drug product by adding large amounts of microorganisms into the preserved compounded preparation to simulate a contamination event during or after compounding. The mixture of microorganisms and preserved drug product are incubated and the number of colony forming units (CFUs) are counted over time.
The added microorganisms include:
- Candida albicans
- Aspergillus brasiliensis
- Escherichia coli
- Pseudomonas aeruginosa
- Staphylococcus aureus
The time points at which the CFUs are counted depends on which of the 4 USP <51> defined categories applies to the product being tested. The goal of this periodic plating is to demonstrate the product’s ability to control microbial growth over time.
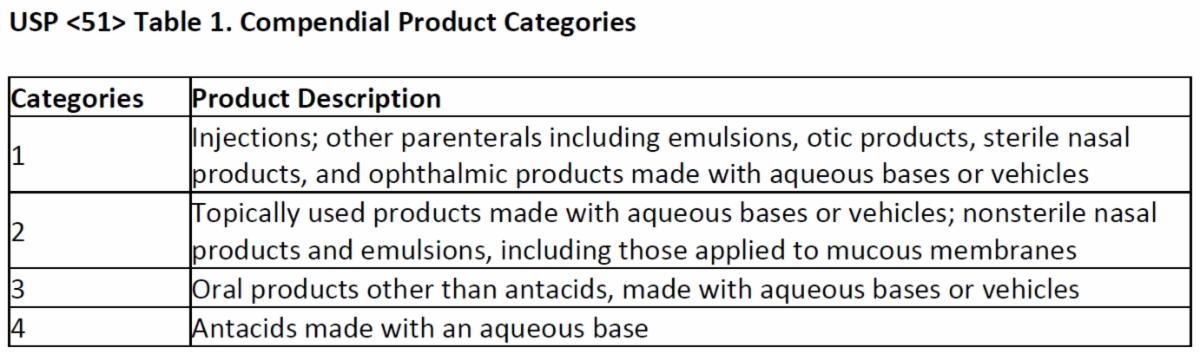
The acceptance criteria to determine if the drug product is sufficiently preserved is also defined in USP <51> based upon the drug category and the type of microorganism.
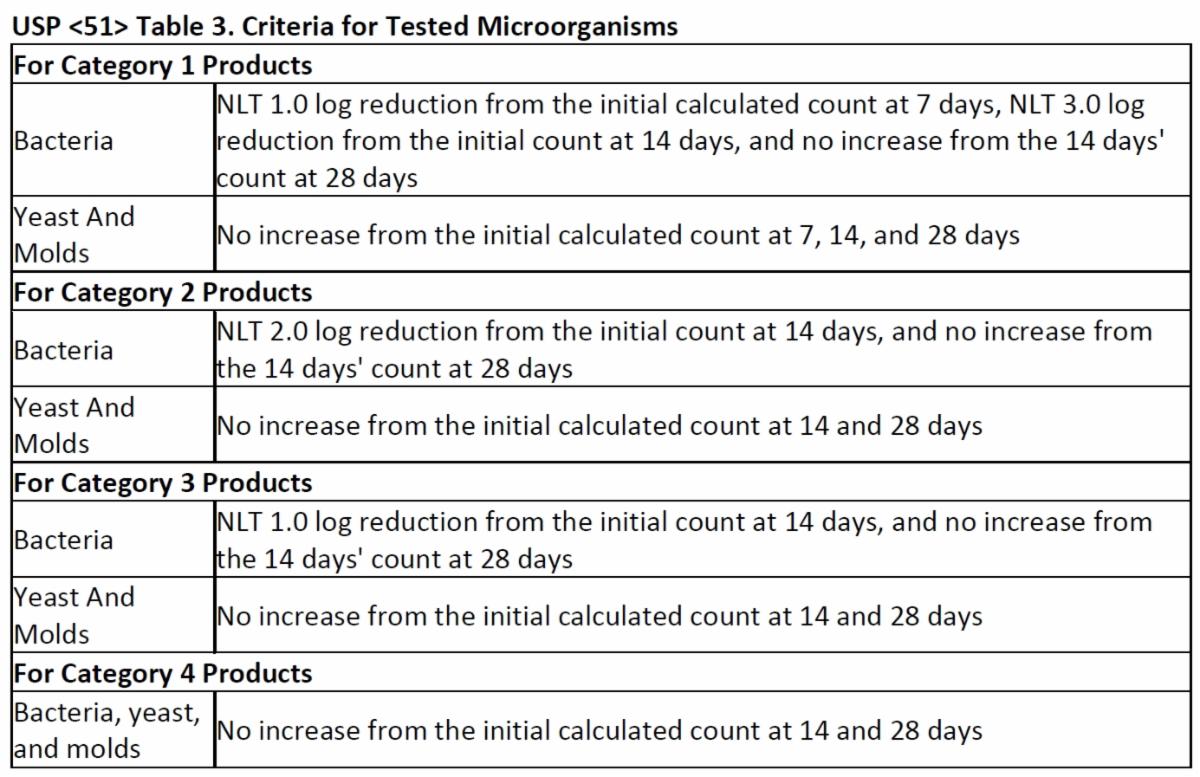
To obtain a passing result, all criteria listed in Table 3 must be met for the applicable drug product category.
For more information on Antimicrobial Effectiveness Testing:
- USP <51> Antimicrobial Effectiveness Testing
- USP <795> Pharmaceutical Compounding Non-Sterile Preparations
- USP <797> Pharmaceutical Compounding Sterile Preparations
- FDA Current Good Manufacturing Practice—Guidance for Human Drug Compounding Outsourcing Facilities Under Section 503B of the FD&C Act
Contact ARL to request a quote info@arlok.com.
